 | P. Díaz-Kruik, D. Roura, E. Hegarty, H. Lehmann, R. Snajdrova, F. Paradisi “Solvent switching in continuous multi-step chemoenzymatic synthesis: telescoping enzymatic synthesis of chiral, pyridine-containing amines with cross-coupling as a case study” OPR&D 2024, in press | 10.1021/acs.oprd.4c00080 |
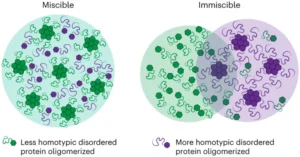 | M. Gil-Garcia, A. I. Benítez-Mateos, M. Papp, F. Stoffel, C. Morelli, K. Normak, K. Makasewicz, L. Faltova, F. Paradisi, P. Arosio “Local environment in biomolecular condensates modulates enzymatic activity across length scales” Nature Communication 2024, 15, 3322 | 10.1038/s41467-024-47435-w |
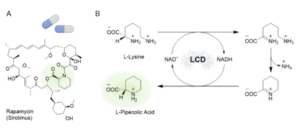 | K. Stalder, A. I. Benítez-Mateos, F. Paradisi “Biosynthesis of L-Pipecolic Acid by a Lysine Cyclodeaminase: Batch and Flow Reactors” ChemCatChem 2024, e202301671 | 10.1002/cctc.202301671 |
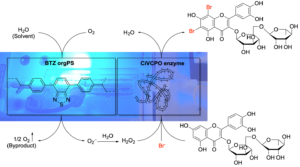 | E. Broumidis, F. Paradisi “Engineering a Dual-Functionalized PolyHIPE Resin for Photobiocatalytic Flow Chemistry” Angew. Chem. Int. Ed. 2024, 63, e202401912 | 10.1002/anie.202401912 |
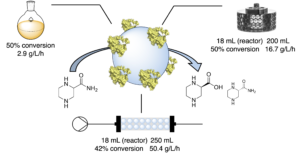 | D. Roura Padrosa, D. Wetzel, S. Hildbrand, P. Tosatti, J. Sedelmeier, K. Puentener, H. Iding, F. Paradisi “Biocatalytic production of (S)- piperazine-2-carboxylic acid: catalyst immobilization and process intensification through continuous flow” Org. Process Res. Dev.2024, 28, 1713-1724 | 10.1021/acs.oprd.3c00347 |
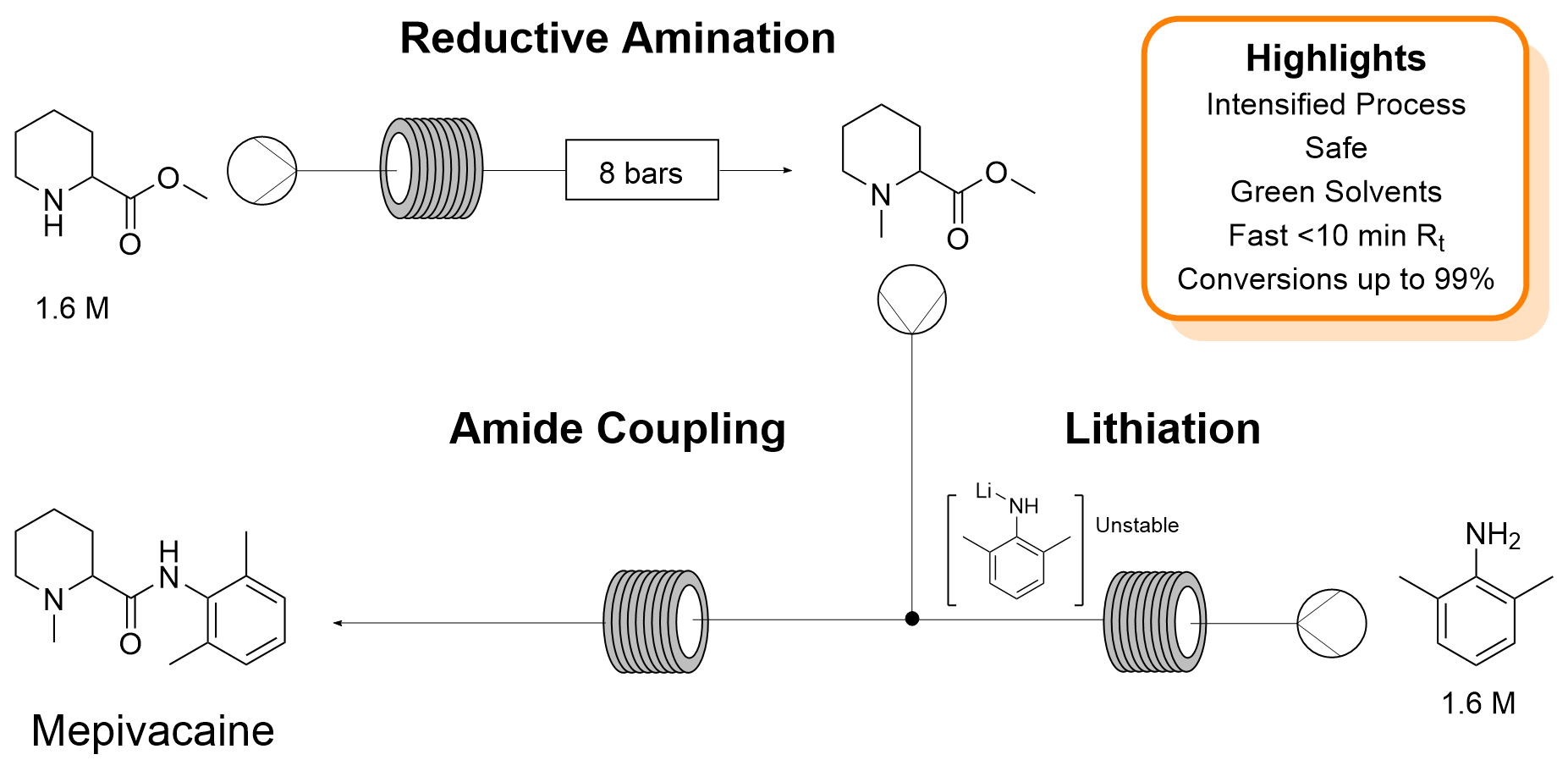 | P. Díaz-Kruik, F. Paradisi “Rapid production of the anaesthetic mepivacaine through continuous, portable technology” Green Chemistry 2024 26, 2313-2321 | 10.1039/D3GC04375D |
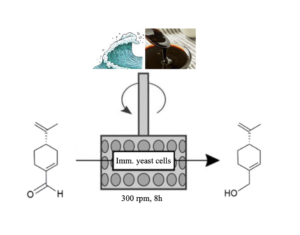 | S. Donzella, C. Compagno, F. Molinari, F. Paradisi, M. L. Contente “Boosting the catalytic performance of a marine yeast in a SpinChem reactor for the synthesis of perillyl alcohol” Reaction Chemistry and Engineering 2023, 8, 2963-2966 | 10.1039/D3RE00474K |
 | A. Mero, N. R. Moody, E. Husanu, A. Mezzetta, F. D'andrea, C. S. Pomelli, N. Bernaert, F. Paradisi, L. Guazzelli “Challenging DESs and ILs in the valorization of food waste: a case study” Frontiers in Chemistry 2023, 11, 1270221 | 10.3389/fchem.2023.1270221 |
 | A. I. Benítez-Mateos, F. Paradisi “Perspectives on Flow Biocatalysis: The Engine Propelling Enzymatic Reactions” Journal of Flow Chemistry 2023, in press | 10.1007/s41981-023-00283-z |
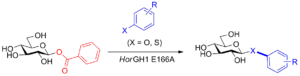 | S. De Lorenzo, L. Pillet, D. Lim, F. Paradisi “Glycosyl Benzoates as Novel Substrates for Glycosynthases” Organic & Biomolecular Chemistry 2023, 21, 6356-6359 | 10.1039/D3OB00979C |
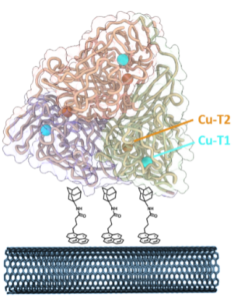 | U. Contaldo, D. Roura Padrosa, H. Jamet, M. Albrecht, F. Paradisi, A, Le Goff “Optimising electrical interfacing between the trimeric copper nitrite reductase and carbon nanotubes” Chemistry - A European Journal, 2023, e202301351 | 10.1002/chem.202301351 |
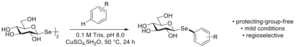 | D. Lim, F. Paradisi “Investigations into the Aqueous Synthesis of Selenoglycoconjugates” European Journal of Organic Chemistry 2023 ejoc.202300496, in press | 10.1002/ejoc.202300496 |
 | S. Gianolio, P. Díaz-Kruik, F. Paradisi “Flow chemistry set up enables integration of chemo- and bio-catalysis” CHIMIA, 2023, 77, 307-310 | 10.2533/chimia.2023.307 |
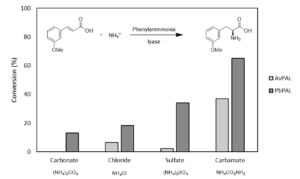 | D. Roura Padrosa, H. Lehmann, R. Snajdrova, F. Paradisi “Sustainable synthesis of L-phenylalanine derivatives in continuous flow by immobilized phenyl ammonia lyase” Frontiers in Catalysis 2023, 3, 1147205 | 10.3389/fctls.2023.1147205 |
 | A. I. Benítez-Mateos, F. Paradisi “Halomonas elongata: a microbial source of highly stable enzymes for applied biotechnology” Applied Microbiology and Biotechnology 2023, 107, 3183-3190 | 10.1007/s00253-023-12510-7 |
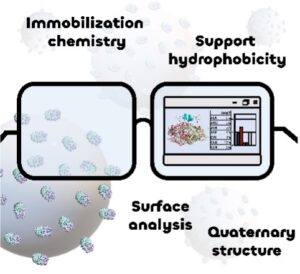 | D. Roura Padrosa, F. Paradisi “Bioinformatic analysis of immobilized enzymes: towards a better understanding of the guiding forces” ChemBioChem 2023, e202200723 | 10.1002/cbic.202200723 |
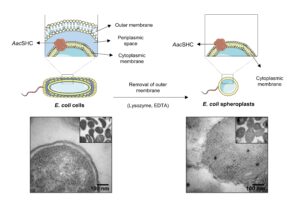 | A. I. Benítez-Mateos, A. Schneider, E. Hegarty, B. Hauer, F. Paradisi “Spheroplasts preparation boosts the catalytic potential of a squalene hopene cyclase” Nature Communication 2022, 13, 6269 | 10.1038/s41467-022-34030-0 |
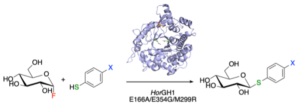 | L. Pillet, N. Almulhim, D. Lim, A. Benítez-Mateos, F. Paradisi “Novel triple mutant of an extremophilic glycosyl hydrolase enables the rapid synthesis of thioglycosides” Chem Commun 2022, 58, 12118-12121 | 10.1039/D2CC04660A |
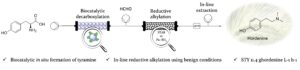 | S. Gianolio, D. Roura Padrosa, F. Paradisi “Combined chemoenzymatic strategy for sustainable continuous synthesis of the natural product hordenine” Green Chemistry 2022, 24, 8434-8440 | 10.1039/D2GC02767D |
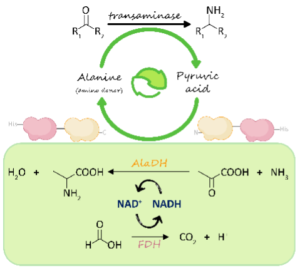 | V. Marchini, A. I. Benítez-Mateos, S. Hutter, F. Paradisi “Fusion of Formate Dehydrogenase and Alanine Dehydrogenase as an Amino Donor Regenerating System Coupled to Transaminases” ChemBioChem 2022, 21, e202200428 | 10.1002/cbic.202200428 |
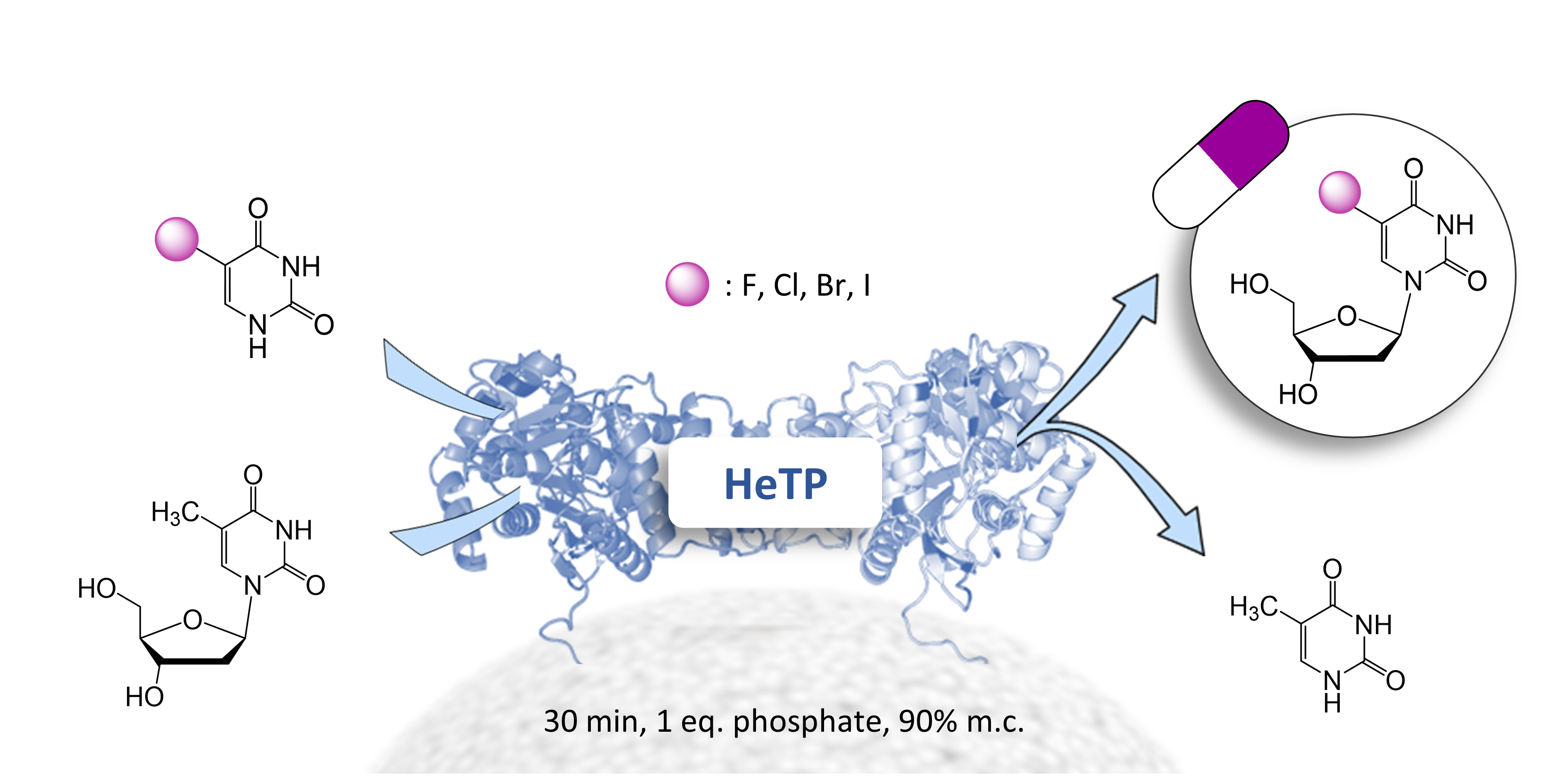 | A. I. Benítez-Mateos, C. Klein, D. Roura Padrosa, F. Paradisi "A novel thymidine phosphorylase to synthesize (halogenated) anticancer and antiviral nucleoside drugs in continuous flow” Catalysis Science &Technology 2022, 12, 6231-6238 | 10.1039/D2CY00751G |
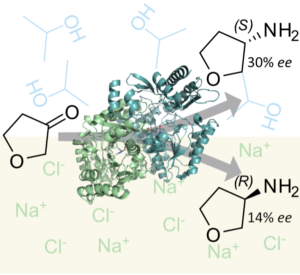 | C. M. Heckmann, L. Robustini, F. Paradisi “Influence of reaction conditions on enzymatic enantiopreference: the curious case of HEwT in the synthesis of THF-amine” ChemBioChem 2022, 23, e202200335 | 10.1002/cbic.202200335 |
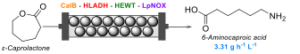 | M. Romero-Fernandez, C. M. Heckmann, F. Paradisi “Biocatalytic production of a Nylon 6 precursor from caprolactone in continuous flow” ChemSusChem 2022, 16, e202200811 | 10.1002/cssc.202200811 |
 | A. I. Benítez-Mateos, D. Roura Padrosa, F. Paradisi “Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses” Nature Chemistry 2022, 14, 489-499 | 10.1038/s41557-022-00931-2 |
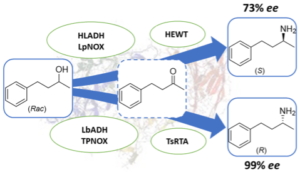 | M. Romero-Fernandez, F. Paradisi “Stereo-divergent enzyme cascades to convert racemic 4-phenyl-2-butanol into either (S)- or (R)- corresponding chiral amine” ChemBioChem 2022 23, e202200108 | 10.1002/cbic.202200108
|
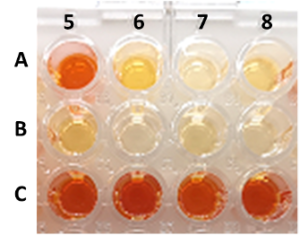 | M. L. Contente, H. Iding, D. Wetzl, K. Puenter, S. P. Hanlon, F. Paradisi “High-throughput screening methods for enzyme-mediated alcohol oxidation” Scientific Reports 2022, 12, 3019 | 10.1038/s41598-022-07008-7 |
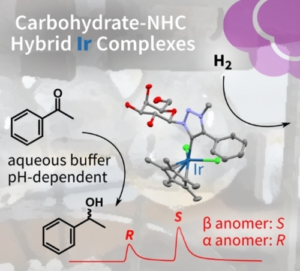 | J. Byrne, L. Delgado, F. Paradisi, M. Albrecht “Carbohydrate-functionalized triazolylidene iridium complexes: hydrogenation catalysis in water with asymmetric induction” ChemCatChem 2022 14, e202200086 | 10.1002/cctc.202200086 |
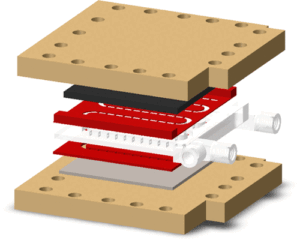 | E. Alvarez, M. Romero-Fernandez, D. Iglesias, R. Martinez-Cuenca, O. Okafor, A. Delorme, A. Conradie, P. Lozano, P. Licence, R. Goodridge, S. Chiva, F. Paradisi, D. Walsh, V. Sans “Enhanced performance of electrochemical baffled continuous flow reactors fabricated with additive manufacturing” ACS Sustainable Chemistry & Engineering 2022, 10, 2388-2396 | 10.1021/acssuschemeng.1c06799 |
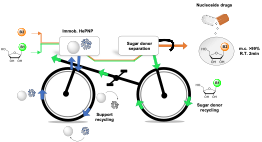 | A. I. Benítez-Mateos, F. Paradisi “Sustainable flow-synthesis of (bulky) nucleoside drugs by a novel purine nucleoside phosphorylase reversibly immobilized” ChemSusChem 2022, 15, e202102030 | 10.1002/cssc.202102030 |
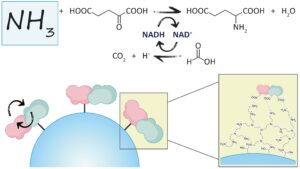 | V. Marchini, A. I. Benítez-Mateos, D. Roura Padrosa, F. Paradisi “Chimeric fusion of glutamate dehydrogenase and formate dehydrogenase yields a bifunctional efficient biocatalyst for the continuous removal of ammonia” Frontiers in Catalysis 2021, 1, art 790461 | 10.3389/fctls.2021.790461 |
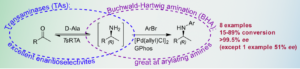 | C. M. Heckmann, F. Paradisi “GPhos ligand enables production of chiral N-arylamines in a telescoped transaminase–Buchwald-Hartwig amination cascade in the presence of excess amine donor” Chemistry- A European Journal 2021, 27, 16616-16620 | 10.1002/chem.202103472 |
 | A. I. Benítez-Mateos, M. L. Contente, D. Roura Padrosa, F. Paradisi “Flow biocatalysis 101: design, development and applications” in Reaction Chemistry & Engineering 2021, 6, 599-611 | 10.1039/D0RE00483A |
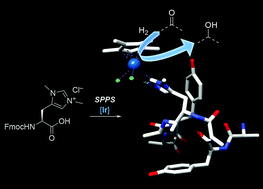 | K. Lenzen, M. Planchestainer, I. Feller, D. Roura Padrosa, F. Paradisi, M. Albrecht “Minimalistic peptidic scaffolds harbouring an artificial carbene-containing amino acid modulate reductase activity” Chemical Communication 2021, 57, 9068 - 9071 | 10.1039/D1CC03158A |
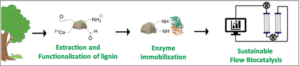 | A. I. Benítez-Mateos, S. Bertella, J. Behaghel de Bueren, J. Luterbacher, F. Paradisi “Dual revalorization of lignin through its use as a versatile and renewable matrix for enzyme immobilization and (flow)bioprocess engineering” ChemSusChem 2021, 14, 3198-3207 | 10.1002/cssc.202100926 |
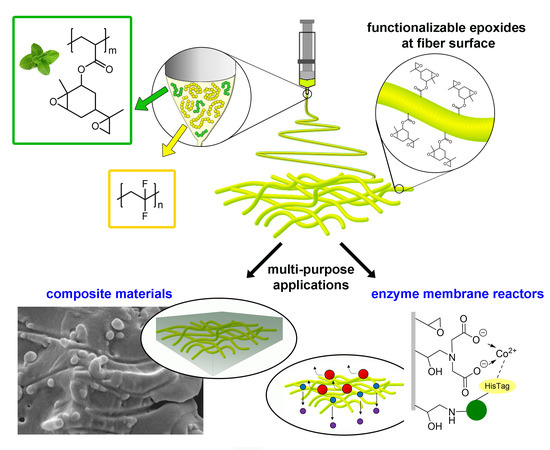 | U. Montanari, D. Cocchi, T. M. Brugo, A. Pollicino, V. Taresco, M. Romero-Fernandez, F. Paradisi, A. Zucchelli, S. Howdle, C. Gualandi “Functionalizable epoxide-rich electrospun fiber based on renewable terpene for multi-purpose applications” Polymers 2021, 13, 1804 | 10.3390/polym13111804 |
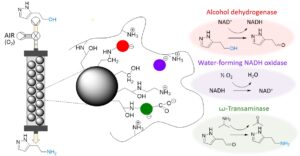 | M. Romero-Fernandez, F. Paradisi “Biocatalytic access to betazole by one-pot multienzymatic system in continuous flow” Green Chemistry 2021, 23, 4595-4603 | 10.1039/D1GC01095F |
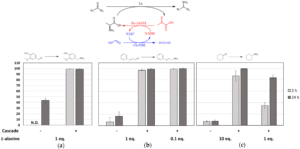 | D. Roura Padrosa, Z. Nissar, F. Paradisi “Efficient amino donor recycling in amination reactions: development of a new alanine dehydrogenase in continuous flow and dialysis membrane reactors” Catalysts 2021, 11, 520 | 10.3390/catal11040520 |
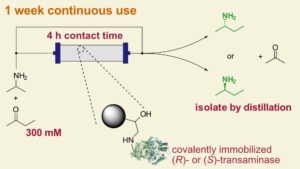 | C. M. Heckmann, B. Dominiguez, F. Paradisi “Enantio-complementary continuous-flow synthesis of 2-aminobutane using covalently immobilized transaminases” ACS Sustainable Chemistry and Engineering 2021, 9,4122-4129 | 10.1021/acssuschemeng.0c09075 |
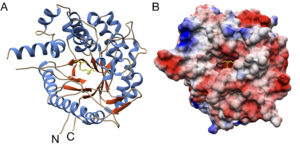 | L. Delgado, C. M. Heckmann, L. J. Gourlay, F. Paradisi “Producing natural vanilla extract from green vanilla beans using a β-glucosidase from Alicyclobacillus acidiphilus” Journal of Biotechnology 2021, 239, 21-28 | 10.1016/j.jbiotec.2021.01.017 |
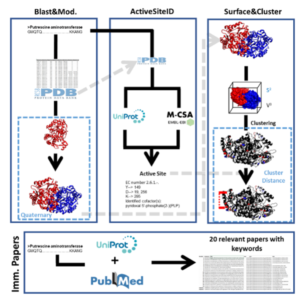 | D. Roura Padrosa, V. Marchini, F. Paradisi “CapiPy: python-based GUI-application to assist in protein immobilization” Bioinformatics 2021, 37, 2761-2762 | 10.1093/bioinformatics/btab030 |
 | L. Delgado, C. M. Heckmann, F. Di Pisa, L. J. Gourlay, F. Paradisi “Release of soybean isoflavones using a β-Glucosidase from Alicyclobacillus herbarius” ChemBioChem 2021, 22, 1223-1231 | 10.1002/cbic.202000688 |
 | M. L. Contente, D. Roura Padrosa, F. Molinari, F. Paradisi “A strategic Ser/Cys exchange in the catalytic triad unlocks an acyltransferase-mediated synthesis of thioesters and tertiary amides” Nature Catalysis 2020, 3, 1020-1026 | 10.1038/s41929-020-00539-0 |
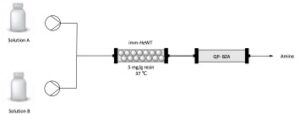 | E. Hegarty, F. Paradisi “Implementation of biocatalysis in continuous flow for the synthesis of small cyclic amines” CHIMIA 2020, 74, 890-894 | 10.2533/chimia.2020.890 |
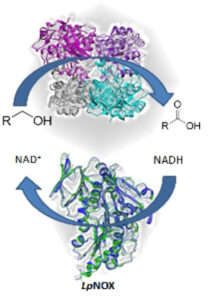 | M. L. Contente, N. Fiore, P. Cannazza, D. Roura Padrosa, F. Molinari, L. Gourlay, F. Paradisi “Uncommon overoxidative catalytic activity in a new halo-tolerant alcohol dehydrogenase” ChemCatChem 2020, 12, 5679-5685 | 10.1002/cctc.202001112 |
 | C. Heckmann, F. Paradisi “Looking back: A short history of enzyme discovery and how they became powerful chemical tools” ChemCatChem 2020, 12, 6082-6102 | 10.1002/cctc.202001107 |
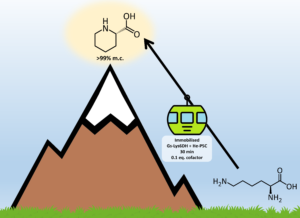 | D. Roura Padrosa, A. I. Benítez-Mateos, L. Calvey, F. Paradisi “Cell-free biocatalytic synthesis of pipecolic acid: a dual strategy approach and process intensification in flow” Green Chemistry 2020, 22, 5310- 5316 | 10.1039/D0GC01817A |
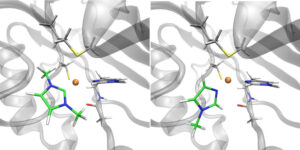 | M. Planchestainer, J. McMaster, C. Schulz, F. Paradisi, M. Albrecht “Carbene-induced rescue of catalytic activity in deactivated Nitrite Reductase mutant” Chemistry: a European Journal 2020, 26, 15206-15211 | 10.1002/chem.202002444 |
 | M. Romero-Fernandez, F. Paradisi “Protein immobilization technology for flow biocatalysis” Current Opinion in Chemical Biology, 2020, 55, 1-8 | 10.1016/j.cbpa.2019.11.008 |
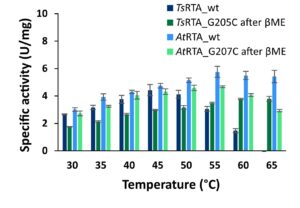 | C. Heckmann, L. Gourlay, B. Dominiguez, F. Paradisi “An (R)-Selective Transaminase from Thermomyces stellatus: Stabilizing the Tetrameric Form” Frontiers in Bioengineering and Biotechnology 2020, 8, art 707 | 10.3389/fbioe.2020.00707 |
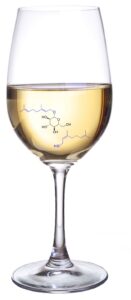 | L. Delgado, M. Parker, I. Fisk, F. Paradisi “Performance of the extremophilic enzyme BglA in the hydrolysis of two aroma glucosides in a range of model and real wines and grape juices” Food Chemistry 2020, 323, 126825 | 10.1016/j.foodchem.2020.126825 |
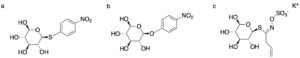 | N. Almulhim, N. R. Moody, F. Paradisi “Engineering novel S-glycosidase activity into extremo-adapted β-glucosidase by rational design” Applied Microbiology and Biotechnology 2020, 104, 4407-4415 | 10.1007/s00253-020-10582-3 |
 | M. L. Contente, L. Tamborini, F. Molinari, F. Paradisi “Aromas flow: Eco-friendly, continuous, and scalable preparation of flavour esters" Journal of Flow Chemistry 2020, 10, 235-240 | 10.1007/s41981-019-00063-8 |
 | R. ul Haque, F. Paradisi, T. Allers "Haloferax volcanii for biotechnology applications: challenges, current state and perspectives" Applied Microbiology and Biotechnology, 2020, 104, 1371-1382 | 10.1007/s00253-019-10314-2 |
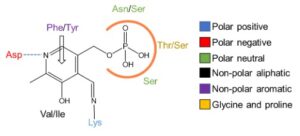 | D. Roura Padrosa, R. Alaux, P. Smith, I. Dreveny, F. López-Gallego, F. Paradisi “Enhancing PLP-binding capacity of class-III ω-transaminases by single residue substitution” Frontiers in Bioengineering and Biotechnology 2019, 7, art 282 | 10.3389/fbioe.2019.00282 |
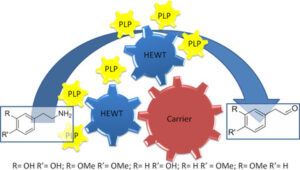 | M. L. Contente, F. Paradisi “Transaminase-catalyzed continous synthesis of biogenic aldehydes” ChemBioChem 2019, 20, 2830-2833 | 10.1002/cbic.201900356 |
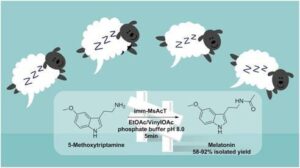 | M. L. Contente, S. Farris, L. Tamborini, F. Molinari, F. Paradisi “Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: a five-minute intensified process” Green Chemistry 2019, 21, 3263-3266 | 10.1039/C9GC01374A |
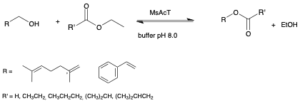 | I. Chiarelli Perdomo, S. Gianolio, A. Pinto, D. Romano, M. L. Contente, F. Paradisi, F. Molinari “Efficient enzymatic preparation of flavor esters in water” Journal of Agricultural and Food Chemistry 2019, 67, 6517−6522 | 10.1021/acs.jafc.9b01790 |
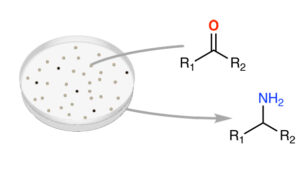 | M. Planchestainer, E. Hegarty, C. Heckmann, L. Gourlay, F. Paradisi “Widely applicable background depletion step enables transaminase evolution through solid-phase screening” Chemical Science 2019, 10, 5952 - 5958 | 10.1039/C8SC05712E |
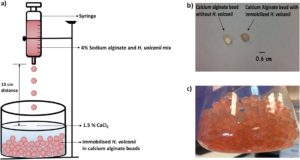 | R. ul Haque, F. Paradisi, T. Allers "Haloferax volcanii as immobilised whole cell biocatalyst: new applications for halophilic systems" Applied Microbiology and Biotechnology 2019, 103, 3807-3817 | 10.1007/s00253-019-09725-y |
 | D. Roura Padrosa, V. De Vitis, M. L. Contente, F. Molinari, F. Paradisi “Overcoming water insolubility in flow: enantioselective hydrolysis of Naproxen ester” Catalysts 2019, 9, 32 | 10.3390/catal9030232 |
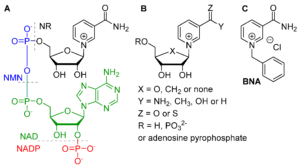 | L. Josa-Culleré, A. S. K. Lahdenperä, A. Ribaucourt, G. T. Höfler, S. Gargiulo, Y. Liu, J. Xu, J. Cassidy, F. Paradisi, D. J. Opperman, F. Hollmann, C. E. Paul “Synthetic biomimetic coenzymes and alcohol dehydrogenases for asymmetric catalysis” Catalysts 2019, 9, 207 | 10.3390/catal9030207 |
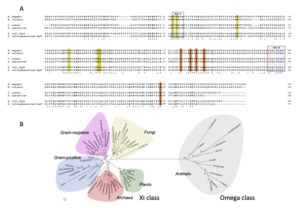 | A. Di Matteo, L. Federici, M. Masulli, E. Carletti, D. Santorelli, J. Cassidy, F. Paradisi, C. Di Ilio, N. Allocati “Structural characterization of the Xi class glutathione transferase from the haloalkaliphilic archaeon Natrialba magadii” Frontiers in Microbiology 2019, 10, art 9 | 10.3389/fmicb.2019.00009 |
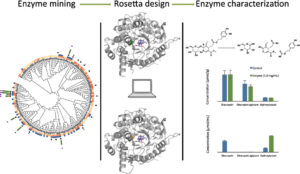 | K. Guggenheim, L. Crawford, F. Paradisi, S. Wang, J. Siegel “b-Glucosidase Discovery and Design for the Degradation of Oleuropein” ACS Omega 2018, 3, 15754-15762 | 10.1021/acsomega.8b02169 |
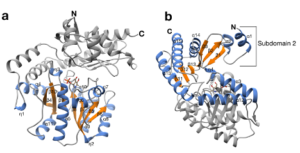 | B. Guidi, M. Plachestainer, M. L. Contente, T. Laurenzi, I. Eberini, L. J. Gourlay, D. Romano, F. Paradisi, F. Molinari “Strategic single point mutation yields a solvent- and salt-stable transaminase from Virgibacillus sp. in soluble form” Scientific Reports 2018, 8, Article number: 16441 | 10.1038/s41598-018-34434-3 |
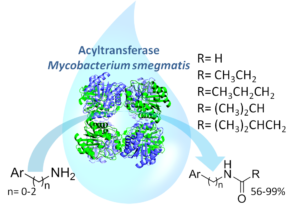 | M. L. Contente, A. Pinto, F. Molinari, F. Paradisi “Biocatalytic N-Acylation of Amines in Water Using an Acyltransferase from Mycobacterium smegmatis” Advanced Synthesis & Catalysis 2018, 360, 4814-4819 | 10.1002/adsc.201801061 |
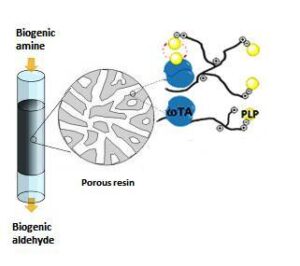 | A. I. Benítez-Mateos, M. L. Contente, S. Velasco-Lozano, F. Paradisi, F. Lopez-Gallego “Self-sufficient flow-biocatalysis by co-immobilisation of pyridoxal 5´-phosphate and ω-transaminases onto porous carriers” ACS Sustainable Chemistry & Engineering 2018, 6, 13151-13159 | 10.1021/acssuschemeng.8b02672 |
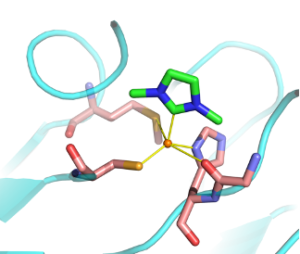 | M. Planchestainer, N. Ségaud, M. Shanmugam, J. McMaster, F. Paradisi, M. Albrecht “Carbene in cupredoxin protein scaffolds: replacement of a histidine ligand in the active site substantially alters copper redox properties” Angewandte Chemie 2018, 57, 10677-10682 | 10.1002/anie.201807168 |
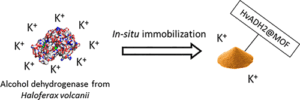 | C. Carucci, L. Bruen, V. Gascon Perez, F. Paradisi, E. Magner “Significant enhancement of structural stability of the hyperhalophilic ADH from Haloferax volcanii via entrapment on metal organic framework support” Langmuir 2018, 34, 8274-8280 | 10.1021/acs.langmuir.8b01037 |
 | M. L. Contente, F. Paradisi “Self-sustained closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions” Nature Catalysis 2018, 1, 452-459 | 10.1038/s41929-018-0082-9 |
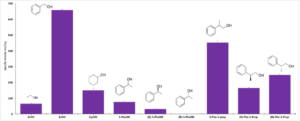 | J. Cassidy, F. Paradisi “Haloquadratum walsbyi yields a versatile, NAD+/NADP+ dual affinity, thermostable, alcohol dehydrogenase (HwADH)” Molecular Biotechnology 2018, 60, 420-426 | 10.1007/s12033-018-0083-6 |
 | M. Planchestainer, D. Roura Padrosa, M. L. Contente, F. Paradisi “Genetically fused T4L acts as a shield in covalent enzyme immobilization dramatically enhancing the rescued activity” Catalysts, 2018, 8, 40 | 10.3390/catal8010040 |
 | L. Tamborini, P. Fernandes, F. Paradisi, F. Molinari “Flow bioreactors as complementary tools for the intensification of biocatalytic processes” Trends in Biotechnology, 2018, 36, 73-88 | 10.1016/j.tibtech.2017.09.005 |
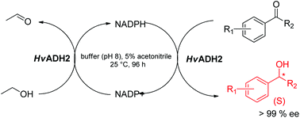
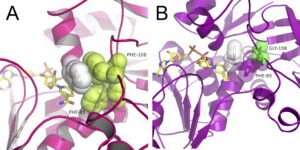 | J. Cassidy, L. Bruen, E. Rosini, G. Molla, L. Pollegioni, F. Paradisi “Engineering substrate promiscuity in halophilic alcohol dehydrogenase (HvADH2) by in silico design” PlosOne 2017, 12, e0187482 | 10.1371/journal.pone.0187482 |
 | D. Alsafadi, S. Alsalman, F. Paradisi “Extreme halophilic alcohol dehydrogenase mediated highly efficient syntheses of enantiopure aromatic alcohols” Organic and Biomolecular Chemistry 2017, 15, 9169-9175 | 10.1039/C7OB02299A |
 | M. L. Contente, F. Dall’Oglio, L. Tamborini, F. Molinari, F. Paradisi “Highly efficient oxidation of amines to aldehydes via flow-based biocatalysis” ChemCatChem 2017, 9, 3843-3848 | 10.1002/cctc.201701147 |
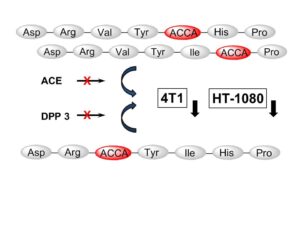 | A. Wester, M. Devocelle, E. A. Tallant, M. C. Chappell, P. E. Gallagher, F. Paradisi “Stabilization of Angiotensin-(1-7) by key substitution with a Cyclic Non-natural Amino Acid” Amino Acids 2017, 47, 1733-1742 | 10.1007/s00726-017-2471-9 |
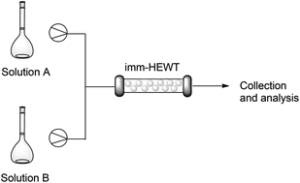 | M. Planchestainer, M.L. Contente, J. Cassidy, F. Molinari, L. Tamborini, F. Paradisi “Continuous flow biocatalysis: production and in-line purification of amines by immobilised transaminase from Halomonas elongata” Green Chemistry 2017, 19, 372-375 | 10.1039/C6GC01780K |
 | M.L. Contente, M. Planchestainer, F. Molinari, F. Paradisi “Stereoelectronic effects in the reaction of aromatic substrates catalysed by Halomonas elongata transaminase and its mutants” Organic and Biomolecular Chemistry 2016, 14, 9306-9311 | 10.1039/C6OB01629D |
| B. Kutasy, F. Friedmacher, L. Pes, D. Coyle, T. Doi F. Paradisi, P. Puri “Antenatal Retinoic Acid Administration Increases Trophoblastic Retinol-Binding Protein Dependent Retinol Transport in the Nitrofen Model of Congenital Diaphragmatic Hernia” Pediatric Resaearch 2016, 76, 614-620 | 10.1038/pr.2015.256 |
| L. Cerioli, M. Planchestainer, J. Cassidy, D. Tessaro, F. Paradisi “Characterization of a novel amine transaminase from Halomonas elongata” Journal of Molecular Catalysis B: Enzymatic 2015, 120, 141–150 | 10.1016/j.molcatb.2015.07.009 |
| H. Takahashi, B. Kutasy, L. Pes, F. Paradisi, P. Puri “Decidual b-carotene-15,15’-oxygenase-1 and 2 (BCMO1,2) expression is increased in nitrofen model of congenital diaphragmatic hernia” Paediatric Surgery International 2015, 31, 37-43 | 10.1007/s00383-014-3621-8 |
| D. Alsafadi, F. Paradisi “Covalent immobilization of alcohol dehydrogenase (ADH2) from Haloferax volcanii: how to maximize activity and optimize performance of halophilic enzymes” Molecular Biotechnology 2014, 56, 240-247 | |
| K. Pandurangan, J. A. Kitchen, S. Blasco, F. Paradisi, T. Gunnlaugsson "Supramolecular pyridyl urea gels as soft matter with antibacterial properties against MRSA and/or E. coli" Chem Commun 2014, 50, 10819-10822 | |
| B. Kutasy, L. Pes, F. Friedmacher, F. Paradisi, P. Puri “Nitrofen increases total retinol levels in placenta during lung morphogenesis in the nitrofen model of congenital diaphragmatic hernia” Paediatric Surgery International 2014, 30, 1017-1022 | |
| B. Kutasy, F. Friedmacher, L. Pes, F. Paradisi, P. Puri “Increased uptake of dietary retinoids at the maternal-fetal barrier in the nitrofen model of congenital diaphragmatic hernia” Journal of Paediatric Surgery 2014, 49, 866-870 | |
| W. Streciwilk, J. Cassidy, F. Hackenberg, H. Müller-Bunz, F. Paradisi, M. Tacke “Synthesis, cytotoxic and antibacterial studies of p-benzyl-substituted NHC-silver(I) acetate compounds derived from 4,5-di-p-diisopropylphenyl- or 4,5-di-p-chlorophenyl-1H-imidazole" Journal of Organometallic Chemistry 2014, 749, 88-99 | |
| K. Robertson, C. Murphy, F. Paradisi “The Synthesis and Biological Testing of Bacilysin Analogues” Amino Acids 2013, 45, 1157-1168 | |
| A. Liliensiek, J. Cassidy, G. Gucciardo, C. Whitely, F. Paradisi “Heterologous overexpression, purification and characterisation of an alcohol dehydrogenase (ADH2) from Halobacterium sp. NRC-1” Molecular Biotechnology 2013, 55, 143-149 | |
| D. Quaglia, M. Pori, P. Galletti, E. Emer, F. Paradisi and D. Giacomini “His-tagged Horse Liver Alcohol Dehydrogenase: Immobilization and application in the bio-based enantioselective synthesis of (S)-arylpropanols” Process Biochemistry 2013, 48, 810-818 | |
| E. O’Reilly, L. Pes, Y. Ortin, H. Müller-Bunz, F. Paradisi “Synthesis of a conformationally constrained delta-amino acid building block” Amino Acids 2013, 44, 511-518 | |
| D. Alsafadi, F. Paradisi “Effect of organic solvents on the activity and stability of halophilic alcohol dehydrogenase (ADH2) from Haloferax volcanii” Extremophiles 2013, 17, 115-122 | |
| F. Hackenberg, G. Lally, H. Müller-Bunz, F. Paradisi, D. Quaglia, W. Streciwilk, M. Tacke “Synthesis and biological evaluation of N-heterocyclic carbene-silver(I) acetate complexes derived from 4,5-ditolyl-imidazole” Inorganica Chimica Acta 2013, 395, 135-144 | |
| L. Timpson, A. Liliensiek, D. Alsafadi, M. A. Sharkey, S. Liddell, T. Allers, F. Paradisi “A comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii” Applied Microbiology and Biotechnology 2013, 97, 195-203 | |
| M. A. Sharkey, J. P. O’Gara, S. V. Gordon , F. Hackenberg, C. Healy, F. Paradisi, S. Patil, B. Schaible, M. Tacke “Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3” Antibiotics 2012, 1, 25-28 | |
| D. Quaglia, J. Irwin, F. Paradisi “Horse Liver Alcohol Dehydrogenase: new perspectives for an old enzyme” Molecular Biotechnology 2012, 52, 244-250 | |
| F. Hackenberg, G. Lally, H. Müller-Bunz, F. Paradisi, D. Quaglia, W. Streciwilk, M. Tacke “Novel symmetrically p-benzyl-substituted 4,5-diaryl-imidazole N-heterocyclic carbene-silver(I) acetate complexes – synthesis and biological evaluation“ Journal of Organometallic Chemistry, 2012, 717, 123-134 | |
| D. Balducci, P. A. Conway, G. Sapuppo, H. Müller-Bunz, F. Paradisi “Novel approach to the synthesis of aliphatic and aromatic alpha-keto acids” Tetrahedron 2012, 68, 7374-7379 | |
| E. Lestini, K. Robertson, C.D. Murphy, F. Paradisi “An alternative mild route to the synthesis of 4-methylenecyclohex-2-enone, a key moiety of the anticancer compounds ottelione A and B” Synthetic Communication 2012, 42,1864-1876 | |
| F. Hackenberg, A. Deally, G. Lally, Sina Malenke,, H. Müller-Bunz, F. Paradisi, S. Patil, D. Quaglia, M. Tacke “Novel non-symmetrically p-benzyl-substituted (benz)imidazole N-heterocyclic carbene-silver(I) acetate complexes – synthesis and biological evaluation” Int. Journal of Inorganic Chemistry 2012, vol. 2012, Article ID 121540, 13 pages. | |
| L. Timpson, D. Alsafadi, C. Mac Donnchadha, S. Liddell, M. A. Sharkey, F. Paradisi “Characterization of alcohol dehydrogenase (ADH12) from Haloarcula marismortui, an extreme halophile from the Dead Sea” Extremophiles 2012, 16, 57-66 | |
| S. Devereux, P. S. Shuttleworth, D. J. Macquarrie, and F. Paradisi “Isolation and Characterisation of Recovered Starch from Industrial Wastewater” Journal of Polymers and the Environment 2011, 19, 971-979 | |
| K. Pandurangan, K. D. Murnaghan, A. Walshe, H. Müller-Bunz, F. Paradisi and G. G. Morgan “Design, Synthesis and Structure of Novel para-quinones and their Antibacterial Activity” Chemical Biology and Drug Design 2011, 78, 787-799 | |
| A. O’Sullivan, D. Balducci, F. Paradisi, K. Cashman, M. J. Gibney, L. Brennan “Effects of supplementation with vitamin D3 on glucose production pathways in human subjects” Molecular Nutrition & Food Research 2011, 55, 1018–1025 | |
| S. Patil, A. Deally, B. Gleeson, F. Hackenberg, H. Müller-Bunz, F. Paradisi, M. Tacke “Synthesis, Cytotoxicity and Antibacterial Studies of Novel Symmetrically and Non-Symmetrically p-Nitrobenzyl-Substituted N-Heterocyclic Carbene- Silver(I) Acetate Complexes” Journal of Inorganic and General Chemistry 2011, 637, 386–396 | |
| S. Patil, A. Deally, B. Gleeson, H. Müller-Bunz, F. Paradisi, M. Tacke."Novel Benzyl-Substituted N-Heterocyclic Carbene–Silver Acetate Complexes: Synthesis, Cytotoxicity and Antibacterial Studies" Metallomics 2011, 3, 74-88 | |
| S. Patil, K. Dietrich, Deally, B. Gleeson, H. Müller-Bunz, F. Paradisi, M. Tacke “Synthesis, Cytotoxicity and Antibacterial Studies of Novel Symmetrically and Non-Symmetrically 4-(Methoxycarbonyl)benzyl-Substituted N-Heterocyclic Carbene-Silver Acetate Complexes” Helvetica Chimica Acta 2010, 93, 2347-2364 | |
| D. O. Frimannsson, M. Grossi, J. Murtagh, F. Paradisi and D. F. O´Shea “Light Induced Anti-microbial Properties of a Brominated BF2 – Chelated Tetraarylazadipyrromethene Photosensitizer” Journal of Medicinal Chemistry 2010, 53, 7337-7343 | |
| S. Patil, J. Claffey, B. Gleeson, H. Müller-Bunz, F. Paradisi, M. Tacke “Synthesis, Cytotoxicity and Antibacterial Studies of Symmetrically and Non-Symmetrically Benzyl- or p- Cyanobenzyl-Substituted N-Heterocyclic Carbene-Silver Complexes” Applied Organometallic Chemistry 2010, 24, 781–793 | |
| K. Pandurangan, S. Gallagher, G. G. Morgan, H. Müller-Bunz, F. Paradisi “Structure and Anti-Microbial Activity of the Silver(I) Complex of 2-Aminophenoxazine-3-one” Metallomics 2010, 2, 530-534 | |
| E. O’Reilly, D. Balducci, F. Paradisi “A stereoselective synthesis of α-deuterium labelled (S)-α-amino acids” Aminoacids 2010, 39, 849-858 | |
| E. O'Reilly; L. Pes; F. Paradisi “From amines to diketopiperazines: a one-pot approach” Tetrahedron Letters 2010, 51, 1696-1697 | |
| S. Patil, J. Claffey, A. Deally, M. Hogan, B. Gleeson, L. M. Menéndez Méndez, H. Müller-Bunz, F. Paradisi, D. Wallis, M. Tacke “Synthesis, Cytotoxicity and Antibacterial Studies of p-Methoxybenzyl-Substituted and Benzyl-Substituted N-Heterocyclic Carbene-Silver Complexes” European Journal of Inorganic Chemistry 2010, 1020-1031 | |
| S. Moran, K. Robertson, F. Paradisi, D. Rai, C. Murphy “Production of lipopeptides in Bacillus sp. CS93 isolated from Pozol” FEMS Letters 2010, 304, 69-73 | |
| P.A. Conway, K. Devine, F. Paradisi “A simple and efficient method for the synthesis of Erlenmeyer azlactones” Tetrahedron 2009, 65, 2935-2938 | |
| E.O’Reilly, E. Lestini, D. Balducci, F. Paradisi “One-step diketopiperazine synthesis using phase transfer catalysis” Tetrahedron Lett. 2009, 50, 1748-1750 | |
| B. Gleeson, J. Claffey, D. Ertler, M. Hogan, H. Müller-Bunz, F. Paradisi, D. Wallis, M. Tacke “Novel organotin antibacterial and anticancer drugs” Polyhedron 2008, 27, 3619-3624 | |
| F. Paradisi, P.A. Conway, A. Maguire, P. C. Engel “Engineered dehydrogenase biocatalysts for non-natural amino acids: efficient isolation of the D-enantiomer from racemic mixtures” Organic and Biomolecular Chemistry 2008, 6, 3611-3615. | |
| D. Giacomini, P. Galletti, A. Quintavalla, G. Gucciardo, F. Paradisi. “Highly Efficient Asymmetric Reduction of Arylpropionic-aldehydes by Horse Liver Alcohol-Dehydrogenase Through Dynamic Kinetic Resolution” Chemical Communications 2007, 4038-4040 | |
| N. Nakazawa, S. Montedonico, H. Takayasu, F. Paradisi, Prem Puri “Disturbance of retinol transportation causes Nitrofen induced hypoplastic lung” Journal of Pediatric Surgery, 2007, 42, 345-349 | |
| F. Paradisi, S. Collins, A. R. Maguire, P. C. Engel “Phenylalanine dehydrogenase mutants: Efficient biocatalysts for synthesis of non-natural phenylalanine derivatives” J. Biotechnol, 2007,128, 408-411 | |
| E. Faulkner, M. Barret, S. Okor, F. Paradisi, P. C. Engel, B. Glennon “The use of fed batch cultivation for achieving high cell densities for the pilot scale production of a recombinant protein (Phenylalanine dehydrogenase) in Escherichia coli” Biotechnology Progress 2006, 22, 889-897 | |
| G. Cainelli, P. Engel, P.Galletti, D. Giacomini, A. Gualandi, F. Paradisi “Engineered Phenylalanine Dehydrogenase in Organic Solvents: Homogeneous and Biphasic Enzymatic Reaction” Organic and Biomolecular Chemistry 2005, 3, 4316–4320 | |
| F. Paradisi, R. Woolfson, K.F. Geoghegan, P.C. Engel “Identification of the Residue Responsible for Catalysing Regeneration of Activity in the Inactive Glutamate Dehydrogenase Mutant D165N” FEBS letters, 2005, 579, 2830-2832. | |
| F. Paradisi, J.L.E. Dean, K.F. Geoghegan, P.C. Engel “Spontaneous chemical reversion of an active site mutation: Deamidation of an asparagine residue replacing the catalytic aspartic acid of glutamate dehydrogenase” Biochemistry, 2005, 44, 3636-3643 | |
| P. Busca, F. Paradisi, E. Moynihan, A. R. Maguire, P. C. Engel “Enantioselective Synthesis of Non-natural Amino Acids using Phenylalanine Dehydrogenases Modified by Site-directed Mutagenesis” Organic and Biomolecular Chemistry 2004, 2, 2684 - 2691 | |
| N. E. Batoux, F. Paradisi, P. C. Engel, M. E. Migaud “Novel Nicotinamide Adenine Dinucleotide analogues as selective inhibitors of NAD+-dependent enzymes” Tetrahedron 2004, 60, 6609-6617 | |
| A. Arcelli, F. Paradisi, G. Porzi, S. Rinaldi, S. Sandri “Acid Hydrolysis of the Ether Bond Assisted by the Neighbouring Amide Group: Effects Induced by Salts and by Structural Changes. Part 6” Journal of Chemical Research (Synopses) 2002, 5, 501-518 | |
| F. Paradisi, F. Piccinelli, G. Porzi, S. Sandri “Enantioselective Synthesis of 2,6-diaminopimelic acid derivatives. Part 3” Tetrahedron: Asymmetry 2002, 13, 497-502 | |
| F. Paradisi, G. Porzi, S. Sandri “A new stereocontrolled synthesis of uncommon tripeptides derived from (2,6)-diaminopimelic acid (2,6-DAP)” Tetrahedron: Asymmetry 2001, 12, 3319-3324 | |
| G. Cainelli, D. Giacomini, P. Galletti, P. Orioli, F. Paradisi “Diastereofacial Selectivity of O-protected-α - Hydroxy Aldehydes: Temperature and Solvent effect”; European Journal of Organic Chemistry 2000, 3619-3626 | |
| F. Paradisi, G. Porzi, S. Rinaldi, S. Sandri “Stereoselective synthesis of α,α’-diaminodicarboxylic acids. Part 2” Tetrahedron: Asymmetry 2000, 11, 4617-4622 | |
| F. Paradisi, G. Porzi, S. Rinaldi, S. Sandri “A simple asymmetric synthesis of (+)- and (-) -2,6-diaminopimelic acids” Tetrahedron: Asymmetry 2000, 11, 1259-1262 | |
| P. Di Felice, M. Maestri, F. Paradisi, G. Porzi, S. Sandri “Highly stereocontrolled boron-mediated synthesis of b-hydroxy-α-aminoacids and dipeptides. Part 2” Tetrahedron: Asymmetry 1999, 10, 4709-4714 | |









































































